Calcium carbonate grinding mill plant
Recommand Crushers:
Algeria is a country which is rich in mineral resources, such as copper,iron, titanium, manganese, gold, and so on. When you want the exploitation of mineral stone, there are many choices. But we provide you with the best combination, improve efficiency and reduce energy Consumption, and we provide mobility to facilitate the migration of the broken program , such as portable crushing plant or mobile crushing plant.
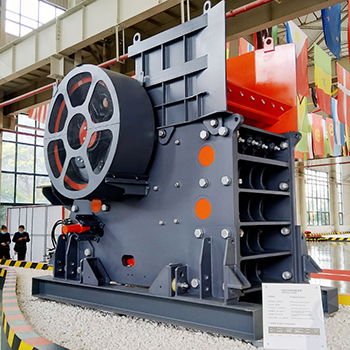
The best crusher combination for calcium :
jaw crusher, jc jaw crusher, hp cone crusher, pfw impact crusher,

INTRODUCTIONS:
Calcium ( /ˈkælsiəm/ KAL-see-əm) is the chemical element with the symbol Ca and atomic number 20. It has an atomic mass of 40.078 amu. Calcium is a soft gray alkaline earth metal, and is the fifth most abundant element by mass in the Earth's crust. Calcium is also the fifth most abundant dissolved ion in seawater by both molarity and mass, after sodium, chloride, magnesium, and sulfate.
Calcium is essential for living organisms, particularly in cell physiology, where movement of the calcium ion Ca2+ into and out of the cytoplasm functions as a signal for many cellular processes. As a major material used in mineralization of bones and shells, calcium is the most abundant metal by mass in many animals.
Notable characteristics
Chemically calcium is reactive and soft for a metal (though harder than lead, it can be cut with a knife with difficulty). It is a silvery metallic element that must be extracted by electrolysis from a fused salt like calcium chloride. Once produced, it rapidly forms a gray-white oxide and nitride coating when exposed to air. In bulk-form (typically as chips or "turnings") the metal is somewhat difficult to ignite, more so even than magnesium chips; but when lit, the metal burns in air with a brilliant high-intensity red light. Calcium metal reacts with water, evolving hydrogen gas at a rate rapid enough to be noticeable, but not fast enough at room temperature to generate much heat. In powdered form, however, the reaction with water is extremely rapid, as the increased surface area of the powder accelerates the reaction with the water. Part of the slowness of the calcium-water reaction results from the metal being partly protected by insoluble white calcium hydroxide. In water solutions of acids, where this salt is soluble, calcium reacts vigorously.
Applications
Calcium is used
as a reducing agent in the extraction of other metals, such as uranium, zirconium, and thorium.
as a deoxidizer, desulfurizer, or decarbonizer for various ferrous and nonferrous alloys.
as an alloying agent used in the production of aluminium, beryllium, copper, lead, and magnesium alloys.
in the making of cements and mortars to be used in construction.
in the making of cheese, where calcium ions influence the activity of rennin in bringing about the coagulation of milk.
PrevPage: Hematite iron ore crusher
Next Page: Granite, basalt and other hard materials' crushing craft made by Kefid